Inhaltsverzeichnis
Nephrotoxic and hepatotoxic effects of a chromium VI compound 1998
Nephrotoxic and hepatotoxic effects of a chromium(VI) compound in comparison to a basic chromium(lll) tanning agent 1998
Nephrotoxic and hepatotoxic effects of a chromium(VI) compound in comparison to a basic chromium(lll) tanning agent 1998
Prof. Dr.Peter C. Bartsch, Dr.Reiner Kimmel,
Prof. Dr.Friedrich W. Schmahl,
Department of Occupational and Social Medicine,
University off Tubingen, Germany
and
Dr Heinz-Peter Germann Lederinstitut Gerberschule, Reutlingen, Germany
Chromium, in the form of its trivalent and hexavalent compounds, is of great industrial interest Specific occupational risks are associated with these compounds, but these risks differ appreciably according to whether the chromium is in its trivalent or hexavalent State.
Trivalent chromium compounds are of low toxicity, but hexavalent compounds are strong oxidants. These not only cause direct tissue damage, but also possess a carcinogenic, mutagenic and teratogenic potential. Besides the lung and the mucous tissue of the nose, the kidney is the main target organ after uptake of Cr(VI).
After oral and/or dermal exposure, the different absorption rates of Cr(III) and Cr(VI) compounds account for the different profiles of toxicity.
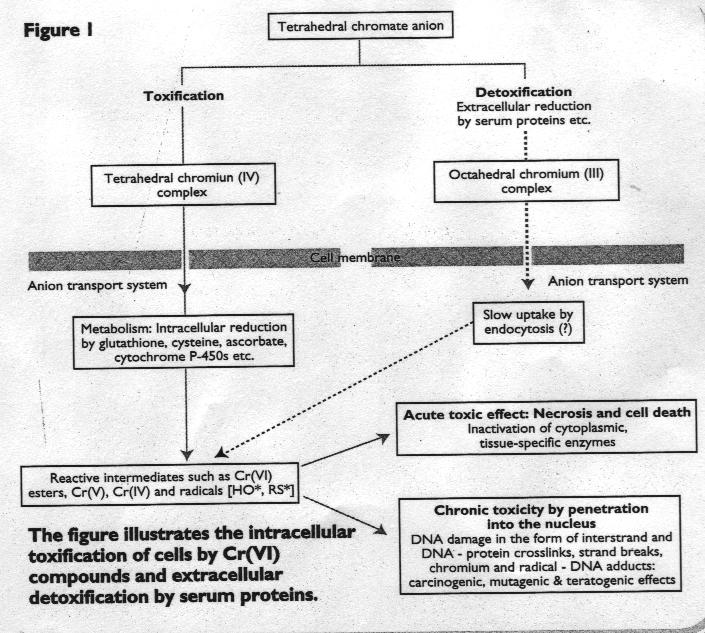
Cr(VI) compounds easily penetrate a cell membrane in the form of the chromate anion.
However, kinetically inert Cr(III) (and most octahedral complexes) cross cell membranes only very slowly.
Once Cr(VI) has crossed the cell membrane it is reduced intracellularly by redoxactive enzymes (e.g. glutathione) and smäll molecules (e.g. ascorbic acid). These reactions form intermediates such as reactive chromium and radical species which attack deoxyribonucleic acid (DNA). The resulting damage (in the form of chromium-DNA adducts, radical adducts, DNA crosslinks, and DNA Strand breaks) interferes with the normal functioning of the DNA studies which have examined the chromium concentration in the urine and various organs of workers who have been exposed to Cr(III) or Cr(VI) by occupation.
Figure 1 and 2
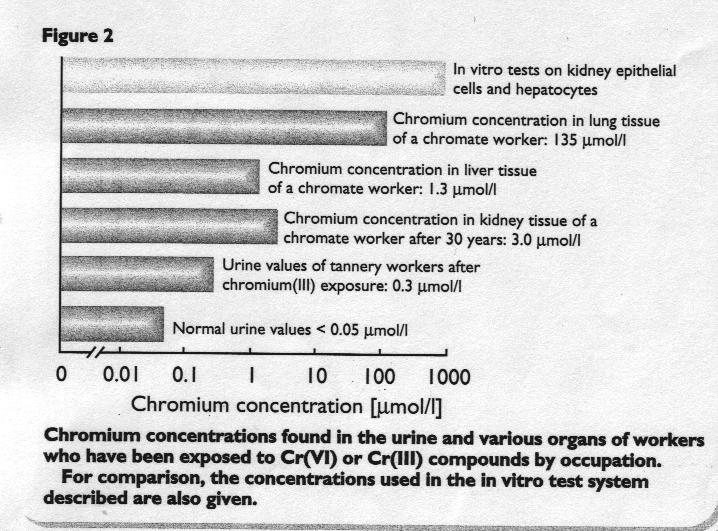
Normal urine values of chromium are below 0.05 umol/l. For tannery workers, a chromium concentration in the urine of approximately 0.3 umol/l has been found.
The chromium concentration in the kidney and liver of a chromate worker was found to be 3 umol/l (normal: 0.3 umol/l) and 1.3 umol/l (normal:0.5 umol/l), respectively. Moreover, in the lung tissue, the chromium concentration was 135 umol/l (normal: 3.7 umol/l).
These findings are presented in Figure 2, with chromium concentrations from in vitro tests for comparative purposes.
It should be remembered, however, that chromium is an essential element and is required for normal carbohydrate and lipid metabolism. Chromium deficiency results in diabetic Symptoms including glucose intolerance, weight loss and cardiovascular diseases.
Study to compare nephrotoxic and hepatotoxic effects
Since the basic mechanisms of cellular absorption, toxicity and detoxification of chromium compounds are not fully understood, the effects a Cr(VI) compound such as K2CrO4 (Merck, Darmstadt, Germany) was studied in comparison to 33% basic Cr(III) sulfate (Chromosal B; Bayer, Leverkusen, Germany).
The investigations focused on the toxic effects on kidney and liver cells (nephrotoxic and hepatotoxic effects), using a novel invitro test system.
In this method chromium compounds were dissolved as sterile 100x stock Solutions in phosphate-buffered saline. This solution was then applied to the culture medium of mitotically active cells for 24 hours.
Prior to chromium exposure and after 24 hours of treatment, the number and volume of the cells were measured with a cell counter and analyser System (Scharfe System, Reutlingen, Germany). In addition, cells were examined after treatment for 24 hours by phase contrast microscopy. Control cells were also grown without chromium exposure.
For the studies presented here, a generally established renal epithelial cell line from the proximale tubule [Opossum Kidney cells(OK)] was used in comparison to a hepatocyte cell line [Human Liver (Hep G2)].
Chromium compounds were tested at concentrations ranging from 0.1 umol/l to 1 mmol/l. In comparison, the float concentration at the beginning of the tanning process is approximately 250 mmol/l (Chromosal B as 2.0% Cr203).
Acute nephrotoxic and hepatotoxic effect of Cr(lll) and Cr(VI) invitro
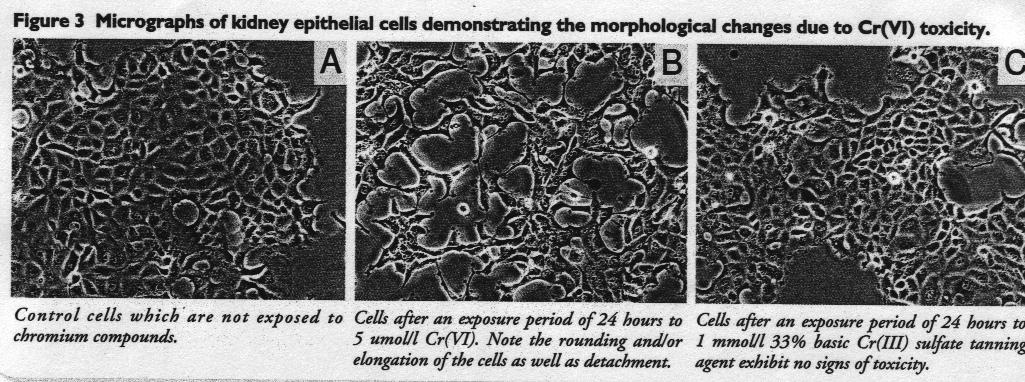
With reference to Figure 3
Treatment with Cr(VI)
At the start of the investigation the control kidney cells (OK) exhibited a polygonal shape with several dark nucleoli within the nucleus (Figure 3A).
Figure 3 and 4
Treatment with Cr(VI) resulted in a rounding and detachment of the cells which was dose-dependent within 24 hours (Figure 3B).
Moreover, cells that were still viable became flattened, with a number of vacuoles or swellings within the region surrounding the nudei, demonstrating the acute toxic effect of Cr(VI).
Although the Cr(VI) concentrations were considerably higher for human hepatocytes(Hep G2), the same morphological alterations were observed as with the OK cells (not depicted).
Treatment with Cr(III)
Cr(III)-treated cells of both kidney and liver cell lines did not show a rounding or detachment even at the highest concentration of 1 mmol/l (Figure 3C). In all experiments, no morphological differences were observed between Cr(III)-treated cells and the corresponding controls.
When measuring the number of viable cells after chromium exposure by using a cell counter, the data confirmed the effects as already seen by microscopic methods.
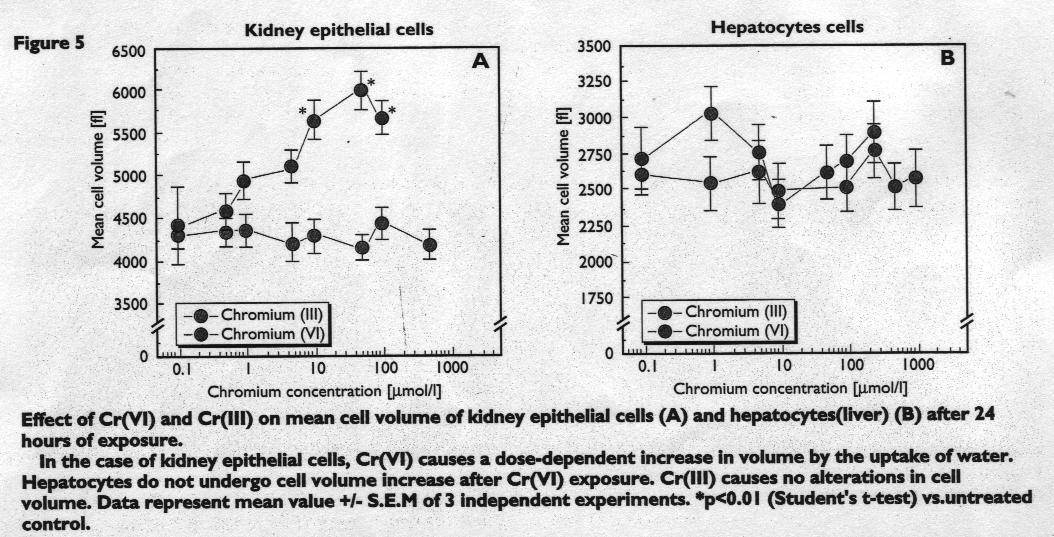
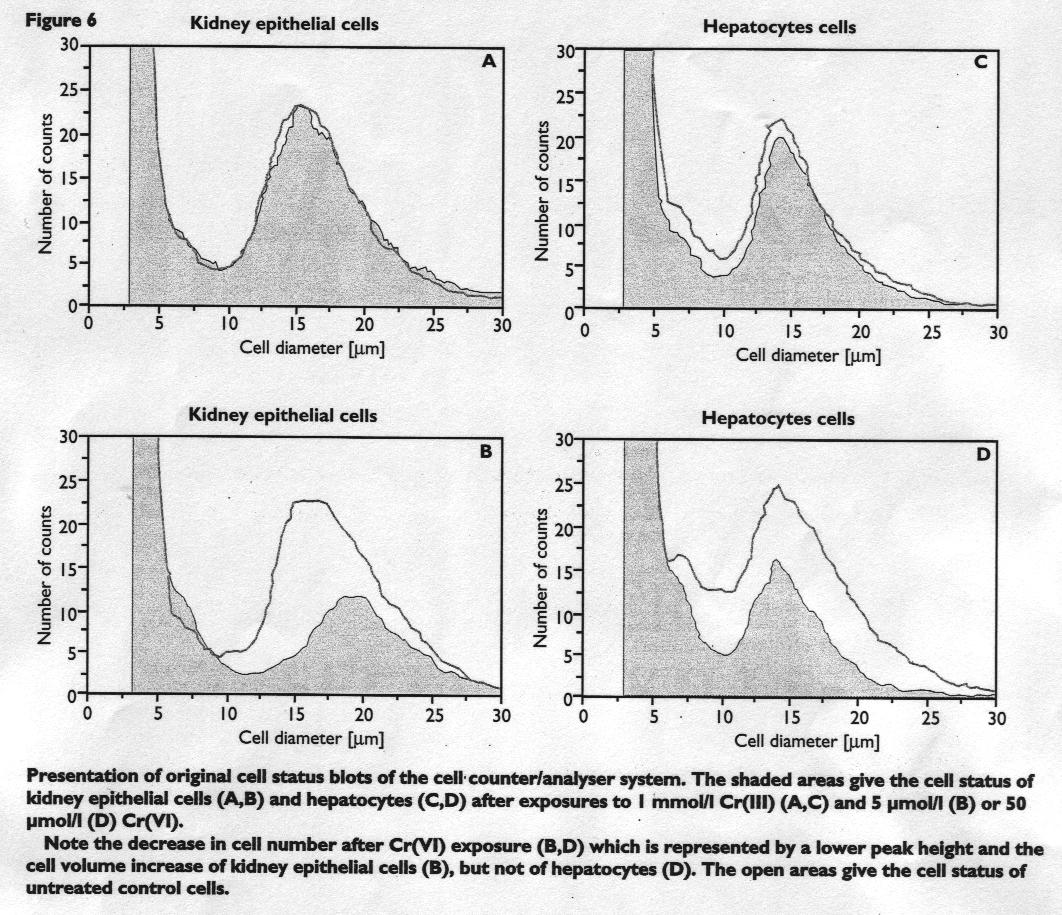
Figure 5 and 6
Exposure to Cr(VI) and effects on cell proliferation
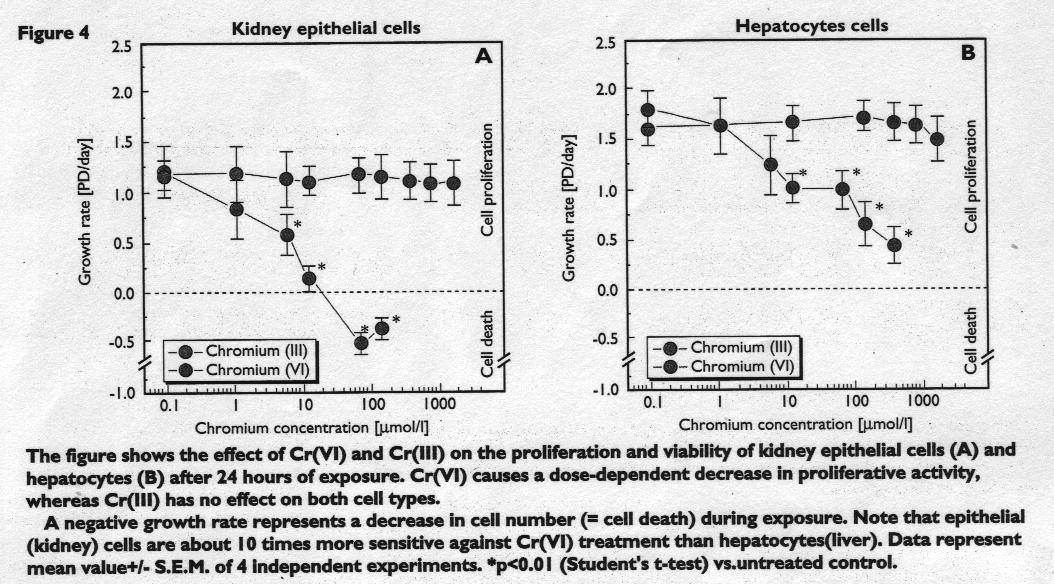
With reference to Figure 4, kidney epithelial cells (OK) and hepatocytes (Hep G2) showed a dose-dependent decrease in growth rate and viability when exposed to Cr(Vl).
As a difference, the effective dose resulting in 50% loss of proliferative activity (=ED50) was 5 umol/l for kidney epithelial cells and approximately 50 umol/l for hepatocytes.
This means, that under the test conditions used in this study, liver cells are about ten times less sensitive against Cr(VI) treatment than kidney cells. This might be because hepatocytes possess enzymatic activities which are able to detoxify the resulting radical species during the reduction to Cr(III) more efficiently than kidney cells. To our knowledge, a hepatotoxic effect of Cr(VI) in humans causing a dysfunction of this organ has not been described yet.
Exposure to Cr(III) and effects on cell proliferation
With reference to Figure 4, the treatment with Cr(III) did not cause alterations in proliferative activity or viability when compared with the controls, thus confirming the low toxicity of the tanning agent.
The effects on Cr(VI) on cell volume
Another finding of this study is that epithelial cell volume increases up to 50% after Cr(VI) treatment, due to water uptake, being dose-dependent. This is shown in Figure 5.
This suggests that Cr(VI)-treated cells undergo necrosis because cell swelling has been shown to be the first step of cell death by the necrotic process. In contrast, a programmed cell death by apoptosis is accompanied by a shrinking of the cells. In this study, both Parameters (cell viability and cell volume) correlate well with each other. This means, that a high toxic effect of a chromium(VI) compound as observed for OK cells, also causes a cell volume increase.
This Cr(VI)-induced increase in cell volume was not observed for hepatocytes. Cr(III) did not alter the cell volume of either OK or Hep G2 cell types.
This finding is summarised in Figure 6 on original cell Status blots.
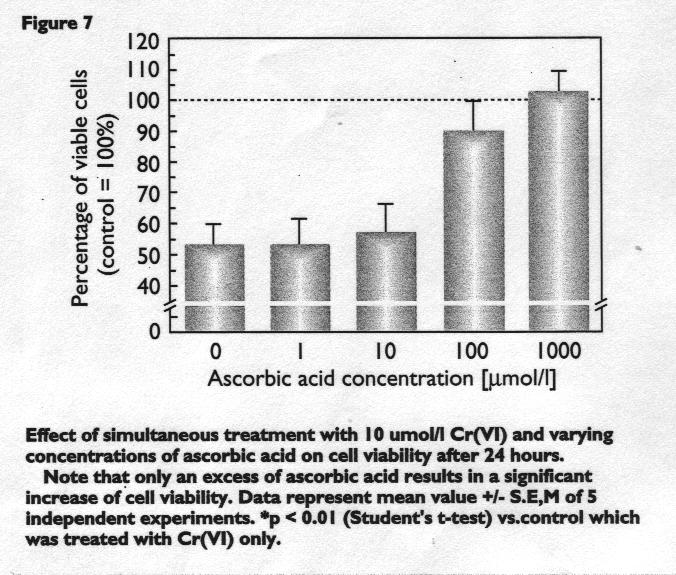
Protective effect of ascorbic acid in vitro
The protective effect of ascorbic acid (vitamin C) for die treatment of Cr(VI) poisoning has been known since 1962 and has been shown in a number of reports.
This is not surprising, however, because ascorbic acid acts as a potent reductant which prompdy reduces Cr(VI) to non-toxic Cr(III) extracellularly. The Cr(III) that is generated is not able to penetrate into the cells within a shorter time period.
As shown for our in vitro model, this protective effect of ascorbic acid is significant at concentrations 10x higher than the Cr(VI) concentration and is presented in Figure 7.
Moreover, a simultaneous admini-stration of Cr(VI) and ascorbic acid is necessary to protect the cells from Cr(VI)-induced damage. A pre-incubation with ascorbic acid prior to Cr(VI) treatment was not effective suggesting that only an extracellular reduction of Cr(VI) to Cr(III) is successful.
Once Cr(VI) has entered the cell and is reduced intracellularly by a reactant such as ascorbic acid or glutathione, reactive intermediates are formed which either attach to DNA or inhibit enzyme activities.
Different toxicity proflies of tri- and hexavalent chromium compounds
The results confirm the different toxicity profile of trivalent and hexavalent chromium compounds and aspects of prevention. A basis is also delivered for future studies on the mechanisms of toxicity of chromium compounds in kidney, liver and other organs (e.g. the lung and mucous tissue).
Moreover, as demonstrated here for chromium compounds, an in vitro-assay might be useful for the examination of other hazardous substances. This may make possible a reduction of animal experiments. Adaptation of the assay to appropriate industrial industries (e.g. leather or chrome industry) might be a useful tool to prove whether non-toxic Cr(III) can be oxidized to toxic Cr(VI) under specific industrial working or processing conditions.
Figure 7
Legend
- Proximal tubular necrosis causing renal failure might be the result of an acute toxification in humans.
- According to the „uptake-reduction“ model.
Acknowledgement
This is an updated and amended version of a paper entided „Nephrotoxic and hepatotoxic effects of a chromium(VI) compound in comparison with a basic chromium(III) tanning agent.“ presented by Prof.Dr. P.C.Dartsch at the Lederinstitut Gerberschule, Reutlingen, Germany in March 1997.
References
- Standeven A.M.,Wetterhahn K.E., Chromium (VI) toxicity: uptake, reduction and DNA damage. JAm.Coll.Toxicol.8, 1275-1283 (1989).
- Wetterhahn K.E., Hamilton J.W., Molecular basis of hexavalent chromium carcinogenicity: effects of gene expression. Sei.Total Environ.86, 113-129 (1989).
- Hyodo K.,Suzuki S.,Furuya N.,Meshizuka K., An analysis of chromium,copper and zinc in organs of a chromate worker.Int. Arch.Occup. Environ.health 46,141-150(1980).
- Anderson R.A.: Chromium metabolism and its role in disease processes in man.clin.Physiol. Biochem.4, 31-41 (1986)
- Buja L.M., Eigenbrodt MX., Eigenbrodt E.H.: Apoptosis and necrosis. Basic types and mechanisms of ceil death. Arch.Pathol.Lab.Med.117, 1208-1214 (1993).
- Korallus U.,Harzdorf C,Lewalter J., Experimental basis for ascorbic acid therapy of poison by hexavalent chromium compounds. Int.Arch.Occup.Environ. Health 53, 247-258 (1984).
- Stearns D.M.,Kennedy L.J.,Courtney K.D., Giangrande P.H., Phieffer L.S.,Wetterhahn K.E., Reduction of chromium (VI) by ascorbate leads to chromium-DNA binding and DNA Strand breaks in vitro. Biochemistry 34, 910-919 (1995).
- Stearns D.M.,Courtney K.D.,Giangrande P.H., Phieffer L.S.,Wetterhahn K.E.,Chromium(VI) reduction by ascorbate: role of reactive intermediates in DNA damage in vitro. Environ.Health Perspect.102(Suppl.3), 21-25 (1994).
Publication:
C. Dartsch, H.-P. Germann, Nephrotoxic and hepatotoxic effects of a chromium(VI) compound in comparison to a basic chromium(lll) tanning agent, World Leather 11, No 3, 5/1998, p. 66-70
Kategorien:
Quellenangabe:
Zitierpflicht und Verwendung / kommerzielle Nutzung
Bei der Verwendung von Inhalten aus Lederpedia.de besteht eine Zitierpflicht gemäß Lizenz CC Attribution-Share Alike 4.0 International. Informationen dazu finden Sie hier Zitierpflicht bei Verwendung von Inhalten aus Lederpedia.de. Für die kommerzielle Nutzung von Inhalten aus Lederpedia.de muss zuvor eine schriftliche Zustimmung (Anfrage via Kontaktformular) zwingend erfolgen.
www.Lederpedia.de - Lederpedia - Lederwiki - Lederlexikon
Eine freie Enzyklopädie und Informationsseite über Leder, Ledertechnik, Lederbegriffe, Lederpflege, Lederreinigung, Lederverarbeitung, Lederherstellung und Ledertechnologie