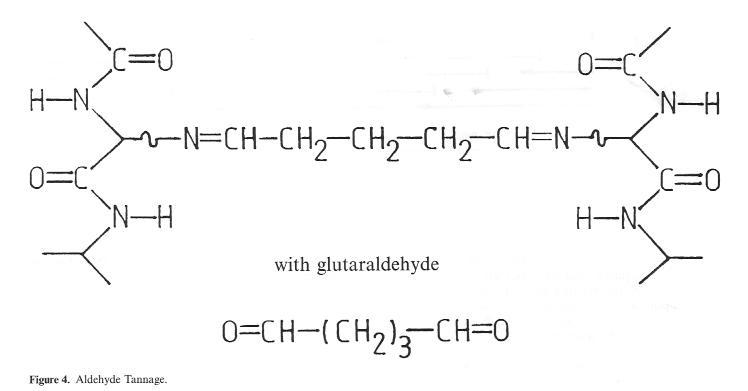Inhaltsverzeichnis
Chrome Tannage From The Viewpoint Of Ecology 1994
H.-P. GERMANN
Westdeutsche Gerberschule, Reutlingen, Germany
Presentedat the 46th South African Convention, 1994.
When we talk about tanning today, the topic is usually raised in connection with environmental compatibility. Due to the increasingly stringent requirements governing tannery effluents as regards their chrome content, and due to a variety of problems involved in the disposal or utilization of by-products containing chrome, chrome tannage in particular has recently come under criticism.
Before entering with enthusiasm into the discussion of possible alternatives to (conventional) chrome tannage, however, I consider it to be useful to first brush up on the fundamental aspects of the theory of hide tannage. This may permit us to outline certain possibil-ities and limitations pertaining to a more environmen-tally sustainable technology before entering into the subject in more detail.
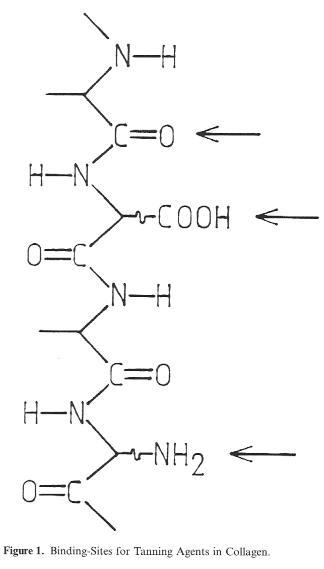
On the basis of its structure alone, the tannable protein substance of native hide material, the Collagen (>90% of the solids), involves several different groups which may be taken into consideration for bonding of the tanning agent: carboxyl groups (as bonding points for mineral tanning agents), (Fig. 1) amino groups (as bonding points for reactive organic molecules such as aldehydes, or for a salt bond with the sulphonic acid functions of syntans), and the peptide group in the backbone of the Collagen (with the C=O oxygen as a bonding point for the hydrogen bridges of vegetable tanning agents). This highly simplified diagram, however, represents only one Single so-called polypeptide chain, of which there are three joined together in screw formation to form a collagen molecule. The collagen molecules form thick bundles called fibrils. These structural units, whose thickness ranges from 20-100 nm (200-1000 Ä), representing a collection of approx. 100-7000 collagen molecules, practically form the basis for the entire structural stability of the hide.
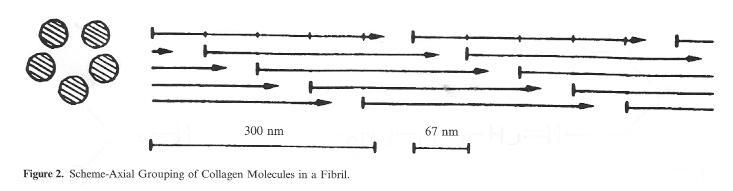
The joined and interwoven collagen fibrils lead to the formation of elementary fibres, and as the result of the next step, of fibres. The basis for this overriding woven network of fibrils, elementary fibres and fibres, tran-scribed frequently by the term „microscopic structure of the hide“ is the so-called micro fibril (Fig. 2) in which rows of collagen molecules are joined together arranged in fives with a circular crosssection. The rod-shaped collagen molecules are offset by around one quarter (67 nm) of their length. Mainly as the result of the inclusion of other substances such as non-leather forming proteins and 2-3% proteoglycans (substances which, together with a protein component, also contain carbohydrates, i.e. sugar), in this tightly packed network of native hide the bonding points are not freely accessible to the tanning agents. It is only in the course of pelt preparation in the beamhouse, particularly in the skin openingup process, that the conditions necessary for good tanning agent penetration and absorption are created. The simultaneous deamidation process of the carbonic acid amide groups which takes place at the same time in the alkaline environment of liming can be viewed as a favourable side effect which leads to the formation of additional carboxyl groups as bonding points for the mineral tanning agents.
Figure 1:
By the term „tannage“, we understand an additional grid-like interlinking of the skin's collagen network, which results in stabilization against heat and micro-organisms and the production of a material which remains supple after drying, retaining the flexibility of the fibre structure.
Chrome tannage is quite obviously particularly suited to obtaining these desirable new properties in the hide material. There can be no other explanation for the 70-80% share held by this method in the main tannage of leather produced around the world and the resulting international Cr tanning agent consumption of approx. 400 000 tons per annum. If we look at alternatives to chrome tannage, quite irrespectively of the question of actual ecological benefits to be drawn from using a different method, it is necessary to consider the extent to which a comparable tanning effect and the achieve-ment of the required characteristics are possible at all on the basis of the existing natural circumstances. To permit Optimum stabilization of the hide material by the tannage process, it is necessary for the tanning agent to penetrate into the interior of the fibrils to cross-link the polypeptide chains. The interstices available between the collagen molecules are in the ränge of approx. 10 Ä (~ 1 nm), while the triple helix of the collagen molecule itself has a diameter of approx. 15 Ä.
Figure 2:
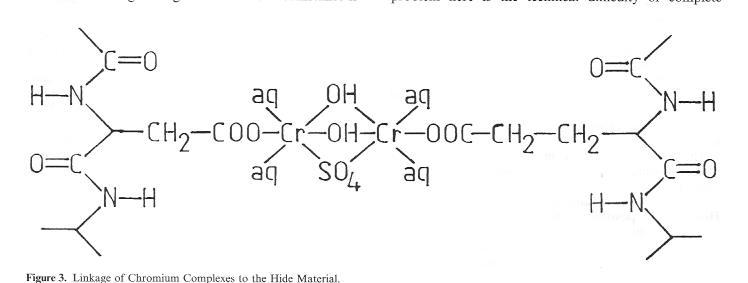
This cross-linking distance can be bridged by, for example, a binuclear chrome complex of the type occurring frequently in tannage floats (Fig. 3). On the other hand, this clearly indicates that larger tanning agent particles have great difficulty in penetrating this area. If we compare Cr tanning agents with other familiär mineral tanning agents such as aluminium, zirconium, titanium or iron, enormous differences become evident. Particularly the marked tendency towards protolysis in aqueous floats is common to all of the named mineral tanning agents with the exception of chrome salts, whose protolysis behaviour is far easier to control. A marked tendency to protolysis means, however, primarily unstable complex salt Solutions, i.e. a rapid increase in particle size and faster achievement of the flocculation point in conjunction with the formation of hydroxide compounds.
Figure 3:
These characteristics of the mineral tannage Systems listed above make diffusion of the tanning agent into the fibrils extremely difficult during the tanning of the hide material. The result can be reaction and deposits on the surface of the fibrils, a process which can be termed a „coating tannage effect“. This circumstance is also the reason for the greater hardness of leather tanned using aluminium, zirconium, titanium or ferrous salts in comparison to chrome tanned leathers.
In addition, the bond created between these tanning agents and the hide is less stable, a circumstance which is reflected in less resistance to washing-out and easier detanning. The reason for the pronounced difference of the described characteristics as against those of chrome lies in the electron structure of the basic ions, and as such there is no prospect of eliminating the problem.
However, a stable, covalent bond is formed with the hide in the case of tanning with aldehydes and similar reactive organic substances as a result of reaction with the amino groups in the collagen (Fig. 4). The fact that the shrinkage temperature of leather tanned in this way hardly, in contrast to the mineral tannage methods described above, exceeds the 80°C mark is indicative of just how far removed this method is from the stabilizing effect of chrome tannage. The extent to which this type of tanning System can assert itself on a broad basis in the future in the field of pre-tannage or intermediate stabilization due to ecological and economic advantages remains to be seen. One of the main benefits hoped for as a result of this type of wet-white process lies in the elimination of chrome shavings, which occur at a quantity of approx. 5-10% of the hide weight as a permanent by-product of the chrome main tannage process. In Germany alone, this adds up to 10000 to 15000 tons per annum, and in the whole of the Single European Market to approximately 100000 tons per annum. However, for the majority of produced chrome shavings, in Germany an excellent recycling possibility exists in the form of leather fibre board production. Alongside this factor, the recovery of protein hydrolysates is also of certain limited importance. However, one problem here is the technical difficulty of complete chrome separation, the other is the resulting chrome cntatining sludge, whose disposal is becoming increasingly difficult. For the wet-white shavings produced as a result of aldehyde tanning, in contrast according to studies not only the recovery of protein hydrolysate but also use in fertilizers
and animal feedstuffs are possible applications, although the latter seems to be placed in doubt by the EC Animal Feeds Act, which prohibits the use of „leather-waste“.
Legal requirements relating to the chrome content of tannery effluent also cause problems in the chrome tannage process.
Figure 4:
A method used when working with a chemical reaction - and tannage must be considered as such, as illustrated here in a highly simplified form, may be considered particularly environmentally friendly when the reactant is transformed almost completely.
Skin + Tanning agent → ← Leather
However, 100% conversion of a reactant is not possible. To allow the complete conversion of at least one of the partners, the quantity of the othjer one is increase, i.e. It is offered in excess. In the case of chrome tannage, this means that there always remains a residual quantity of substance in the tannage float. By improving the reaction conditions, for example by increasing the concentration via a shorter float and increasing the final temperature and pH, it is possible to enhance the conversion process and reduce the quantity of surplus chrome tanning agent, provided this is permitted by the leather quality.
The alternative method, in other words offering the hides in surplus, is naturally completely impractical, as hide areas which are not completely tanned ccannot be simply separated, making the entire product unusable.
However, one possibility exists of offering toher carboxyl groups in addition to the hide material, for example in the form of dicarboxylic acids which can act as a link between smaller chrome complexes and so also effect a sufficient cross-linking of the collagen even when working with a markedly lower tanning agent surplus.This fact is brought to bear when using the „high exhausting chrome tanning process“.
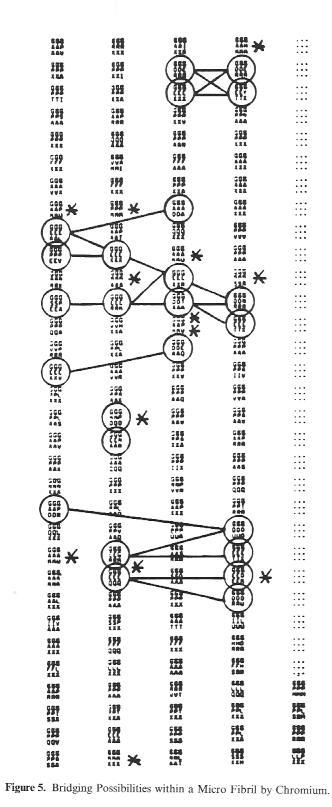
If we look at this schematic drawing of a microfibril section over a length of 96 amino acid residues (i.e. 1/10th of the length of a single collagen molecule), the possibilities for cross-linking through chrome tannage become clear. The circled (tripeptide) sections contain the carboxyl groups for the tanning agent bonding.The connecting lines mark the various pissibilities for cross-linking by chrome.
Assuming that on average one Cr atom falls to each carboxyl group during tanning, whhich corresponds to the bonding of all groups by binuclear Cr complexes, the total of 222 carboxyl groups per collagen molecule produce a chrome oxide content of approx. 4-5% found in practice do not deviate far from this calculation.
A somewhat different method of improved tanning agent utilization is the deliberate formation of additional carbopxyl groups in the hide material. The conversion of the amino groups with an aldehyde carbonic acid hs proven feasible here. The additionally available carboxyl groups produced as a result of locatlization of the amino groups undergoing conversion in the same sections of the collagen with the natural carboxyl groups of the aspartate and glutamate result in mpore effective exhaustion of the offered chrome tanning agent. This allows a reduction of the offered chrome oxide in some cases of up to 1%.
Despite high exhaustion tanning methods and a low offer of chrome tanning agent, the remaining residual chrome content of the tannage float sill reneubst too high b far to permit it to be simply released into the public sewage system, for example in Germany without preliminary effluent treatment. Alongside common effluent treatment together with the process floats produced during the post-tanning processes, chrome recycling of residual tannage floats and sammying floats presents an interesting alternative, particlarly for large scale tanneries. Irrespective of this, however, there will still remain at least a chrome containing sludge from the treatment of the post tanning effluent-even if a wet-white material undergoes, for example, only a chrome posttanning process.
For these sludge deposits and also for the leather of all kinds containing chrome, a method of utilization or disposal must be found which is both ecologically and economically acceptable. The most promising method would appear to be that of combustion or rather „thermal recycling“, which primarily guarantees a drastic reduction of the waste volume. According to research by Prof. Schwedt in Germany, it is possible to avoid release of the dreaded Chrome (VI) into the atmosphere during this combustion process. A Joint research project by the British and Danish Leather Institutes and the Westdeutsche Gerberschule promises to shed light on this question in the near future and to test possibilities for the recycling of combustion residues containing chrome.
Finally, it must be stated that despite the criticism which has been aimed at chrome tannage for various reasons, to date no actual scientific proof has been produced of the alleged negative influences on the environment or on human health-although Chrome (III) tanning agents have been in use for the past 100 years.
The type of tanning process which will be considered the cleanest and most environmentally sustainable in the future will only emerge in the light of future developments and through the compilation of appro-priate ecological balance sheets. However, our endeav-ours in the Service of the product „Leather“, i.e. its Standing and its position, should be aimed towards maintaining a wide variety of tannage methods - provided they are ecologically acceptable-to allow the best solution to be selected in the fulfilment of an increasing ränge of requirements placed on this beautiful material.
Figure 5:
Publication:
H.-P. Germann, Chrome Tannage From The Viewpoint Of Ecology, JSLTC 79, 1994, p. 82-85
Kategorien:
Quellenangabe:
Zitierpflicht und Verwendung / kommerzielle Nutzung
Bei der Verwendung von Inhalten aus Lederpedia.de besteht eine Zitierpflicht gemäß Lizenz CC Attribution-Share Alike 4.0 International. Informationen dazu finden Sie hier Zitierpflicht bei Verwendung von Inhalten aus Lederpedia.de. Für die kommerzielle Nutzung von Inhalten aus Lederpedia.de muss zuvor eine schriftliche Zustimmung (Anfrage via Kontaktformular) zwingend erfolgen.
www.Lederpedia.de - Lederpedia - Lederwiki - Lederlexikon
Eine freie Enzyklopädie und Informationsseite über Leder, Ledertechnik, Lederbegriffe, Lederpflege, Lederreinigung, Lederverarbeitung, Lederherstellung und Ledertechnologie