Inhaltsverzeichnis
The Evolution of the Unhairing Process as Influenced by Technological, Economic and ecological considerations 1997
The 1997 John Arthur Wilson Memorial Lecture: The Evolution of the Unhairing Process as Influenced by Technological, Economic and ecological considerations
by
Heinz-Peter Germann
Lederinstitut Gerberschule Reutlingen (LGR)
Erwin-Seiz-Strasse 9, D-72764 Reutlingen, Germany
Introduction
For generations of tanners the beamhouse processes and especially the liming, unhairing processes and the opening up of the Collagen fiber structure have been considered the most important operations in leather manufacture as so far as the quality and texture of the finished leather are concerned. Indeed, the results of improper liming methods can hardly be compensated by modifications of the bating or tanning process.
More and more the character of leather is influenced by a great variety of post tanning and finishing operations, nonetheless, major faults originating in the beamhouse'such as drawn grain, grain wrinkles or unremoved hair, cause trouble not only at this stage, but the grain damage, loose grain and lack of opening up becoming visible in the dry condition and are of decisive influence with regard to the leather quality.
On the other hand it is well known, that the unhairing process of hides and skins, including the removal of the hair and epidermis as well as the opening up of the hide, are the most polluting operations in the tannery. Although there have been important developments and improvements made in the past few decades, we are still looking for a more desirable, cleaner unhairing technology.
Since the time of John Arthur Wilson a lot of progress has been achieved in the first major step in leather production including the reduction of processing time and even of effluent loadings. These developments only became possible because of a deeper understanding of the properties of keratin and collagen. In particular in the differences in chemical behaviour between these two kinds of proteins.
The studies of J. A. Wilson and his contemporaries on the Separation of hair and skin have to be recognized as pioneering work in this respect.
Unhairing Processes
SWEATING
The sweating method of unhairing skins, probably the oldest method known for unhairing, consists of allowing soaked and fleshed hides or skins to remain for 1-2 days in a warm and humid place to cause hair loosening. Subsequently, the skins are dumped into saturated lime water for up to 24 hours to stop the bacterial process and to get a slight swelling of the skin. Unhairing is effected mechanically. Unfortunately, this simple unhairing method may cause serious skin damage, since it is very difficult to control the process with to respect of temperature, humidity and Ventilation.
In 1917, Wilson and Daub investigated the sweating process on fresh sheep skin kept in a closed receptacle with an atmosphere saturated with water vapour at about 38°C. They studied the destruction of cells and the Separation of the epidermis under the microscope.2 After one day they noticed a very pronounced odor of ammonia, which together with other amines that were being evolved, assisted in the unhairing action. At the same time, the Separation of cells of the Malphigian layer were the first action that became visible under the microscope. On the second day the epidermis, glands and wool were completely separated firom the derma and the Malphigian layer (the layer of youngest cells adjoining the true skin) cells had almost completely disintegrated. The corneus layer was still intact At the end of 42 hours, the wool could be easily rubbed off and the skin was undamaged.
LlMING
Anolher very old method of unhairing (liming) is based on the observation that a solution of lime can be used safely to separate hair firom skin with relatively little damage to the skin. In his book 'Modem Practice in Leather Manufacture' published in 1941, Wilson points out that the reason for this is the limited solubility of lime (appr. 1,5 g/l) causes a pH value of about 12,5 in a saturated solution at 21°C.
Because of the great practical impoitance of the reaction of lime water on skin to the tanner, Wilson and Daub in 1924 made a microscopic study of the stracture of calf skin during 7 months contact with saturated lime water at 20°C. For comparison, in a second test series, the lime water was covered with a layer of toluene to be sure to avoid bacterial growth. As both series gave exactly the same test results and proved to be sterile for at least 6 months, the changes occuring in the skin were definitely not caused by bacteria.
The first noticeable action was the disintegration of cells of the Malphigian layer of the epidermis. After 5 days this action permitted the easy removal of hair and epidermis. After the third week the glands had been attacked and nearly all epidermal cells surrounding the hair had disappeared. At the end of the fifth week Wilson and Daub noticed the complete disappearance of the elastin fibers. The corneus layer of the epidermis and the hair began to disintegrate slowly after 15 weeks and had disappeared after 7 months. The collagen fibers remained sharply defined for 5 months, after which they began to assume a glassy appearance. This was due to a slow hydrolysis that resulted in a heavy loss of collagen after 7 months.
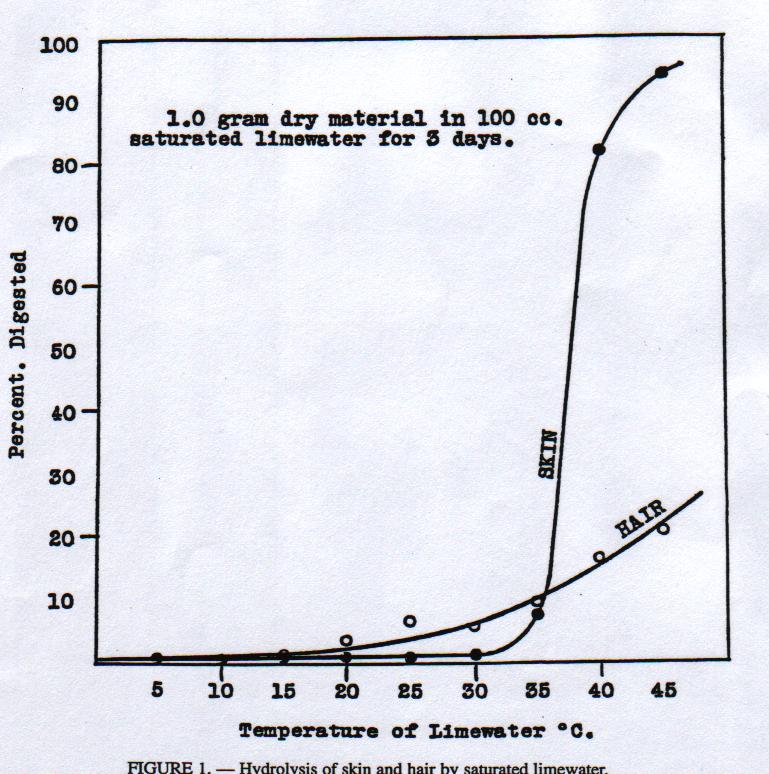
During the same period they also studied the influence of temperature on the hydrolysis of hair and skin (Fig. l). For both types of protein an increase in digestion was observed with increasing temperature of the saturated lime water. However, the hair was more rapidly digested than skin at temperatures up to 35°C, whilst the skin was much more rapidly digested than hair above 35°C.
Figure 1
In his book 'Modern Practice in Leather Manufacture' Wilson commented on this: „This information is of utmost importance to the practical tanner, but he must be cautioned against assuming that safety lies only in liming at low temperatures. If lower temperatures are used, the time of liming to get easy unhairing is increased, and a low temperature may result in more harm than a higher one that is carefully controlled.“ This observation is one more confirmation of his far-sightedness in recognizing the importance of proper process control.
Using fresh lime liquors hair loosening takes about one week and it is well known that the process is accelerated by using old (used) lime liquors. This is because old lime liquors are enriched in ammonia, amines, bacteria and enzymes ihat assist in hair loosening. In addition swelling is reduced due to the presence of other skin decomposition products.
The essential negative point to consider in using old lime liquors is the risk of uncontrolled bacterial growth, especially in non-saturated liquors.' This led to the introduction of the paddle wheel, to stir up the undissolved lime to main-tain Saturation and to assist diffusion of the lime into the hides as well.
Hair-Destroying Process
Looking for a more rapid way of unhairing and a reduction in the loss in Collagen led to the addition of sharpening agents. In particular sulphides became commonly used. This represented the first essential Step in improving the control of the process to conserve collagen and to specifically attack the keratin molecules in the hair. Thereby the reaction time of hair loosening could be shortened to 1-3 days. In addition to sodium sulphide and sodium hydro-sulphide, the most widely used sharpening agents today, calcium sulphide and arsenic sulphide were also used. Of course the latter was completely replaced a long time ago for reasons of health.
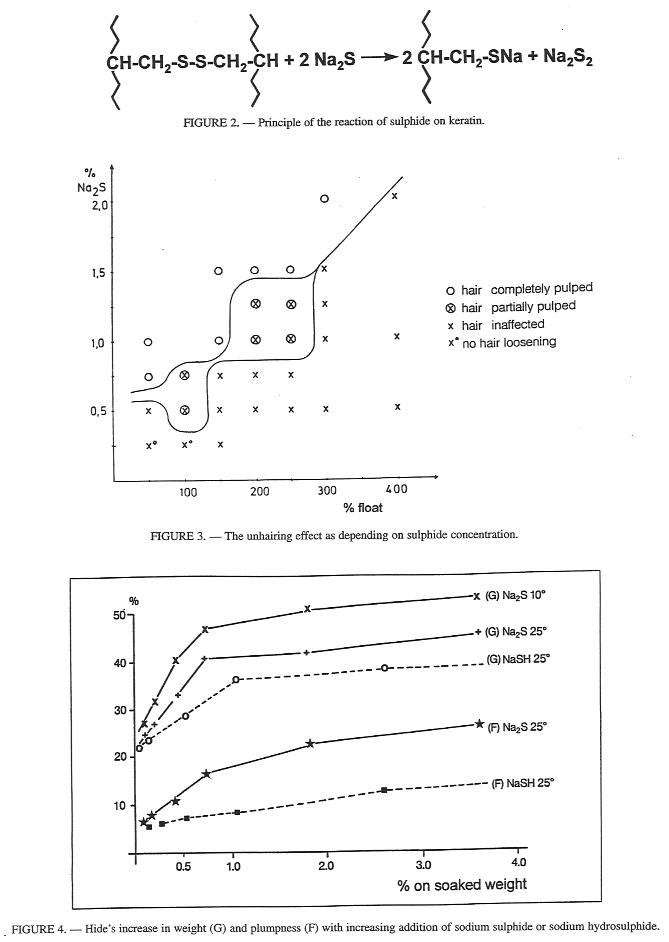
The essential finding that sulphide is bound rapidly by keratin in contrast to collagen, while alkali is bound slowly by both types of protein, is chemically based on the attack of the disulphide crosslinks in keratin (Fig. 2), This results in the Splitting of the hair into individual keratin molecule by a hydrolytic decomposition. The unhairing effect of sharpened lime liquors depends on the concentration of sulphide as early studies of Chambard revealed in 19307 on a static liming System. It was clearly shown by Smidek and Heidemann only 10 years ago (Fig. 3). From a 0,5% sodium sulphide concentration upwards a hair-destroying attack is noticeable.
When Wilson first developed a hair-destroying method for speeding up leather production during the World War he used about 8% of sodium sulphide (60%) in a float of approximately 300% water in a paddle vat to achieve pulping and largely dissolving of the hair within 6 hours. This was followed by treatment with a calcium Chloride liquor of the same concentration over night.
In the post-war period the rapid hair-destroying liming method has become the most widely used Standard procedure. This happened as a consequence of economic considerations that including leather quality improvement, all cases where the recovered hair lost its value or where the reduction in processing time overcompensated the loss of hair. Normally, a paddle or drum liming System uses 2-4% hydrated lime (60%) and 2-3% sodium sulphide. In 16-20 hours at room temperature these chemicals achieve adequate hair-destroying, scud loosening and removal of epidermis.6 However, for the production of soft aniline leathers a more intensive opening up of the hide substance and a more complete removal of scud and hair roots is required. In this case, the lime-sulphide-system must be adjusted to produce a lower degree of swelling and plumpness. The influence of swelling and plumpness in the liming process on the physical parameters and characteristics of the leather as well as their dependence upon the different liming chemicals was investigated extensively by Herfeld and Schubert at Reutlingen in the 1960s (Fig. 4). They demonstrated that measuring of the hide's increase in weight is not sufficient to assess liming behaviour since as the weight gain does not always correlate with the change in thickness and plumpness of the hide.
These findings led to an increased use of liming Systems based on mixtures of hydrated lime, sulphide and hydrosul-phide. Replacing sulfide with hydrosulphide was shown to reduce the swelling of the hide. According to the experimental results of the same authors swelling and plumpness can also be controlled by the temperature and the float volume, with higher temperatures causing less swelling and an increased opening up. Exceptionally low float volumes of < 30% produces little or no swelling thereby speeding up the penetration of liming chemicals. When using liming Systems of the last-mentioned type, also referred to as 'drum painting' processes, the hair-destruction is completed most more rapidly as a result of the high concentration of unhairing chemicals. Nevertheless, a subsequent liming after the addition of water is required to effect an adequate opening up.
Figure 2, 3 and 4
Rapid Hair-Saving Process
The new hair-destroying processes using higher concentra-tions of sulphides, became the world-wide Standard procedure for unhairing within a few decades. Attention was then tumed to the development of new liming Systems •to protect the environment which included reduced water consumption, the reduction or replacement of sulphide and the reduction in COD loading of the effluent.
As a matter of course it is necessary that any new System does not result in deterioration of pelt or leather quality. A significant reduction in water consumption was achievable by replacing the rinsing processes by washing processes and decreasing the float ratio. The recycling of liming wash liquors is a practical possibiüty to minimize the water consumption, however, the recycling of lime liquors is not without problems concerning leather quality, because of the inconsistancy of soluble components.
It was also mainly for environmental reasons that the hair-saving process of unhairing was rediscovered within the last 10-15 years. The SIROLIME process and a number of subsequenüy developed commercially available hair-saving Systems are based on the principle of a partial immunization of the hair. The essential advantages that a hair-saving process offers, are the drastic reduction in COD and sludge load in the effluent as well as reducing amount of sulphide required. In the original SIROLIME process developed by Scroggie et al. in Australia the hides were impregnated with hydrosulphide, briefly washed and the hydrosulphide remaining in the hair oxidized by the addition of hypochlo-rite. The addition of lime activated the remaining sulfide in the region of the hair roots to Start keratolysis which results in hair loosening. The hair is recovered by filtration and the pelts subjected to a lime-sulphide reliming Step for destroy-ing the residual hair and opening up the fiber structure. A number of problems, such as like the evolution of hydrogen sulphide during the impregnation Step and unsatisfactory hair and scud removal, had to be overcome by process modifications. Subsequently, a number of other hair-saving Systems based on the principle of immunization have been developed.
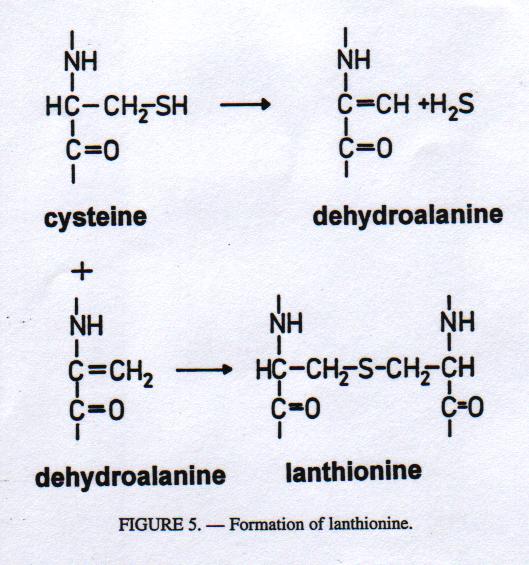
Under the influence of sufficiently strong alkali the disulphide cross-links (cystine) formed by cysteine residues linking individual keratin molecues in the hair are transformed into a thioether called lanthionine. This compound can no longer be split reductively by sulphides (Fig. 5).19 This reaction mainly takes place in the hair shafts, whereas the hair roots remain susceptible to sulphide because of the delay and a much lower degree of immunization. However, problems may result in such a System from exceeding the reaction time or temperature that may cause incomplete removal of short hair and epidermis. The problem was solved by the development of a so-called 'controlled immunization' as described by Christner.16 By the application of a liming auxiliary with hydrolytic and reductive activity penetrating into the region of the hair roots before the immunization Step, the hair in its prekeratinous region is protected from a stronger immunization. After the addition of lime the immunization is effected at the outer hair only.
Figure 5
In a subsequent step smaller amounts of strong reducing agents like sodium hydrosulphide or sodium sulphide are added whereupon hair loosening is achieved within 30 minutes. The largely intact hair is rubbed off and recovered from the float by filtration. Then water and lime are added to effect the opening up of the hide structure. Besides an excellent quality of the pelts and the resulting leather, these modern hair-saving unhairing technologies result in substantial environmental benefits in particular in reductions in sludge volume and effluent loads (BOD, COD, NH4-N, suspended solids) depending on the type of hides and the actual process conditions.
Low-Sulphide Systems
In search of sulphide-free or low-sulphide unhairing Systems a number of organic thio-compounds with reducing activity including thioglycollate, thiourea derivatives and in particular mercaptoethanol have been proposed. While the reaction of mercapto-alcanols with keratin is similar to that of the inorganic sulphides, it is important to pay attention to their much more rapid oxidation. On the other hand, the rapid oxidation of mercaptoethanol is an advantage with respect to the effluent, as it is completely oxidized at the end of the unhairing process into oxidation products that are not toxic. If these thio-compounds are used exclusively, the evolution of toxic hydrogen sulphide in the beamhouse processes is impossible.
For reasons of economics these thio-compounds are usually used in conjunction with inorganic sulphides. This also results in cleaner pelts because of a longer lasting reducing activity in the process, and the remaining ecolog-ical advantages of a low-sulphide System.
As mentioned previously, hair loosening in old (used) lime liquors improved as the content of ammonia and amines increased. Once this was known the unhairing effect of amines alone became a subject for study.
While the hair loosening effect of ammonia is only moderate, methylamine and in particular dimethylamine (DMA) proved have good depilatory action. Amines can effect hair loosening below 35°C without any additional chemicals but it is not a hair-destroying process. For practical results they were used in combination with alkali. Depending on the concentration and temperature the dimethylamine/sodium hydroxide System fcould be applied to both hair-saving and hair-destroying processes. In the American leather industry especially dimethylamine (DMA) and dimethylamine sulfate (DMA) was used for
many years until it was removed from the market because of its capacity to form carcinogenic nitrosamines under certain conditions. Today we are aware of the fact that volatile secondary amines react with nitrous gases devel-oped in combustion processes, as e.g. by a gasoline driven forklift, to form stable nitrosamines.
The non-volatile and highly water-soluble ethanolamines that are not suspected of forming nitrosamines are still being used as auxiliary liming agents in combination with alkali and sulphides. However, the secondary amine, diethanolamine should not be used as there may be a risk of nitrosamine creation. These amines, as well as the above mentioned thio-compounds, are useful auxiliary agents to control swelling and immunization in State of the art hair-saving processes.
Enzyme Unhairing
A number of leather scientists have proposed that air-saving enzymatic unhairing methods will someday replace sulfide unhairing as an economical and ecological sound alternative. So it seems that the wheel is Coming full circle - the oldest method of hair loosening, after some improvements, would become the latest one. As early as 1910, O.Röhm had patented the unhairing with pancreas tryptases aiming at the replacement of the almost uncontrollable sweating method of unhairing by a much more controllable process. The proteolytic attack of hair roots and epidermis was found to be effective only after pretreatment with alkali swelling or some other treatment to slightiy break down the epidermis thereby enabling enzyme penetration. The second condi-tion found was the need for an incubation time of more than 12 hours. This is at least partially due to the fact that there are no specific enzymes available to disintegrate hair roots and epidermis. The long processing time becomes a great disadvantage because even low collagenolytic activity in the technical preparations of the proteolytic enzymes for unhairing can cause damage to the grain.
This is especially true of hide regions previously damaged by bacterial or mechanical action. In addition a serious problem for enzymatic unhairing is the incomplete removal of short hair, in particular when the processing time is limited in order to avoid grain damage. For this reason at the present time the use of some sulphide in enzymatic unhairing processes is indispensable. Nevertheless, research on enzymatic unhairing is on going. At LGR, for instance, an optimized unhairing process using a protease of low molecular weight at neutral pH-conditions is currently under investigation.
Oxidative Unhairing
„ Wool researchers in 1950 discovered that the cleavage of disulphide crosslinks in keratin from wool can be effected by oxidation. A practical unhairing process using chlordioxide, generated by sodium chlorite in an acidic float, was first proposed by Rosenbusch in 1964. Although the process was optimized with respect to chemicals and time consumption, two disadvantages of this System remainded. First the addition of alkali after 4 hours of treatment resulted in a rapid dissolution of hair and epidermis. The second disadvantage is related to worker health and safety. There is also an increased cost factor involved. Motivated by reducing both environmental pollution and processing time, large-scale technical experiments were carried out by Müller and Krings in Eastern Germany using hydrogen peroxide liming in an alkaline media.33 This liming System requires only two hours for unhairing and opening up leads to bleached and scud-free pelts.
However, the process is more expensive than lime-sulphide unhairing and must be carried out in stainless-steel or resincoated vessels as wooden drums are attacked by the process. It is largely for this latter reason that the oxidative unhairing process has not yet been further adopted.
Continuous Processing
There have also been developments towards the rationalization and improvement of working conditions by through-feed unhairing processes. In the USA the automation of soaking and liming processes was investigated using a conveyor belt System connecting two soaking vats and one liming vat containing a sodium sulphide liquor. It was found that a reaction time of 10 minutes per step was suficient to achieve a hair-destruction. The removal of the epidermis and the opening up of the hide structure subsequently had to be accomplished batch-wise in a liming vessel.
In Germany, the 'Darmstadt Process' has been introduced by Heidemann, Dorstewitz and Sagala using hide by hide processing. Fresh or soaked hides hanging over bars hair-side up, were unhaired by spraying with a sodium-sulphide solution followed by stripping of the hair-pulp. An advantage of this System is the application of the sulphide on the grain preventing contamination of the flesh side. Here for the first time the chemical reaction of unhairing was combined with the mechanical processes of fieshing and Splitting in a process line.
More recently, in USA the principle of spraying with sulphide has been used in a pilot plant for an unhairing of cattle after stunning, prior to the removal of the hide. Since the direct discharge to the waste treatment facilities at a packing plant is not feasible, the problem of waste treatment from the unhairing process remains to be solved.
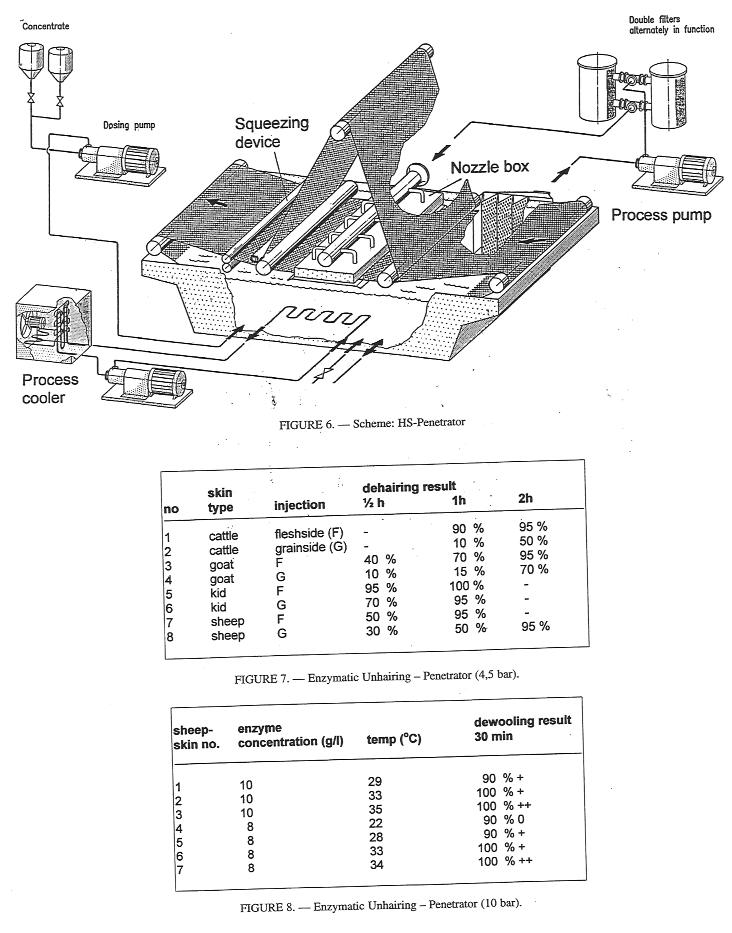
At West German Tanners' School WGR (now: LGR) Reutlingen in 1980 another approach to a throughfeed System based on the high pressure injection of process chemicals was started. The development beginning with experiments on the use of inoculation guns ended-up in the 'High Speed Penetrator' constructed by J. Krause GmbH, Hamburg, as described by Petersen. In the High Speed Penetrator a process pump generating a pressure of up to 10 bar is used to effect injection of the unhairing liquor via a System of nozzle tubes into the hides that are transported continuously to the injection or impregnation zone by means of a conveyor belt (Fig. 6). Excess float is subsequently removed by a squeezing device. Concerning the unhairing process it is of interest that enzymatic hair loosening can be speeded up drastically by the injection method, which results in a reduction of the risk of undesirable side-effects. Depending on the type of skin, experiments showed that a reaction time of 0,5-3 hours at a temperature of >30°C was required to achieve satisfactory hair loosening (Fig. 7). In general, injection from the flesh side yielded better results than injection from the grain side. Experimental data indicate that the best unhairing results were achieved at temperatures of 33-35°C (Fig. 8). At lower temperatures the enzyme activity is decreased and temperatures higher than 35°C may cause problems in the quality of the pelts, as well as in the long-term stability of the enzymes.
In any case, the enzyme unhaired skins need an additional reliming process for opening up the fiber structure and to guarantee complete removal of short hair. For reasons of cost and some technical limitations, in particular concerning the use on bovine hides, this process is - just as the other approaches to throughfeed processing mentioned - not in commerical use at present.
CONCLUSION
Today, from a technological and ecological point of view low-sulphide Systems using either organic thio-compounds or amines (except the secondary ones), sometimes in conjunction with enzymes, have to be considered as the best technologies existing for unhairing. For commercially acceptable results in all those Systems the additional use of some sulphide is still required.
It is merely depending on regional and individual conditions concerning effluent-related cost and possibilities of hair utilization, as well as on the technical equipment available, wether these Systems should be used in a hair-destroying or a hair-saving process for the best economic benefit.
Looking in the fiiture for a completely sulphide-free unhair-ing method, I see principally two possibilities: an improved enzyme process with a better dififusion enhancing the selectivity or an oxidative unhairing based on the experiences with an alkaline peroxide System.
In either case, these Systems will be far more sensitive to small changes in process parameters. For such a System to be successful more accurate and improved process control Systems, in the spirit of John Arthur Wilson, will be a must.
Figure 6,7 and 8
References
- CA. Money; JSLTC 80,175,1996.
- J. A. Wilson; Modern Practice in Leather Manufacture,Reinhold Publishing Corporation, New York, 201-235,1941.
- J.A. Wilson and G. Daub; Ind. Eng. Chem. 16, 602,1924.
- H.B. Merrill; IndEng. Chem. 16, 1144, 1924.
- H.B. Merrill; Ind. Eng. Chem. 17, 36, 1925.
- A. Zissel; Bibliothek des Leders 2, Umschau-Verlag,Frankfurt/Main, 1988.
- Chambard; Cuir techn. 19,188,1930.
- J. Smidek and E. Heidemann; Das Leder 38, 48,1987.
- H. Herfeld and B. Schubert; Das Leder 14, 77,1963.
- H. Herfeld and B. Schubert; Das Leder 14, 117, 1963.
- H. Herfeld and B. Schubert; Das Leder 17, 25,1966.
- H. Herfeld and B. Schubert; Leder- und Häutemarial 19(45), 667 and 19(49), 722,1967.
- H. Herfeld and B. Schubert; Leder- und Häutemarkt 21(46), 709 and 21(50), 759,1969.
- R.W. Cranston, M.H. Davis and JG. Scroggie; /. Soc.Leath. Technol Chem. 70, 53,1986.
- R.W. Cranston, CA. Money and DJ. Abbott; Proc. XX IULTCS Congress, Philadelphia, 1989.
- J. Christner; Das Leder 41, 177, 1990.
- T. Blair, Leather Manufacturer 104, 18, 1986.
- J. Christner; JALCA 83, 183, 1988.
- E. Heidemann; Fundamentals of Leather Manufacturing, Eduard Roether KG, Darmstadt, 165-216, 1993.
- F. Elsinger, K.H. Münz, R. Babinek and V. Olip; Das Leder 38, 143(1987).
- V. Olip; Leder- und Häutemarkt 42(11), 1 and 42(20),1, 1990.
- F. Knaflic; Das Leder 23, 157, 1972.
- G.D. McLaughlin; JALCA 22, 345, 1927.
- E.K. Moore; JALCA 27, 2,1932.
- D.G. Bailey, R.C. Doerr, W. Fiddler and S.H.Feairheller; JALCA 77,476,1982.
- O. Röhm; Enzym. Unhairing, Patent 1910 DBP1026038.
- O. Röhm; Collegium 1913, 371.
- H. Wolf; Das Leder 42, 260, 1991.
- H.-P. Germann and H. Wolf; Leder- und Häutemarkt 45(14), 7, 1993.
- P. Alexander and C.F. Earland; Nature 166, 396,1950.
- K. Rosenbusch; Das Leder 15, 119, 1964.
- K. Rosenbusch; Das Leder 16, 237, 1965.
- L. Müller and M. Krings; Das Leder 42, 68, 1991.
- W.K. Heiland, M. Komanowsky, N.C. Aceto and J. Craig; JALCA 78, 267,1983.
- M. Komanowsky, W.K. Heiland, G.E. Senske, N.C. Aceto and J. Craig; JALCA 78, 300, 1983.
- E. Heidemann, R. Dorstewitz and J. Sagala; Das Leder 28, 138, 1977.
- E. Heidemann; Das Leder 30,1, 1979.
- R. Dorstewitz and E. Heidemann; Das Leder 30, 185,1979.
- E. Heidemann; Das Leder 35,143,1984.
- D.G. Bailey; World Leather 8(3), 43,1995.
- A. Petersen and H.-P. Germann; Das Leder 40,187 and 205, 1989.
- W. Pauckner; Leder- und Häutemarkt 44(35), 1, 1992.
- H.-P. Germann; Leather 193, 33, 1991. JALCA, VOL 92, 1997
Publication:
H.-P. Germann, The 1997 John Arthur Wilson Memorial Lecture: The Evolution of the Unhairing Process as Influenced by Technological, Economic and ecological considerations, JALCA 92, 6/1997, p. 84-92
Kategorien:
Quellenangabe:
Zitierpflicht und Verwendung / kommerzielle Nutzung
Bei der Verwendung von Inhalten aus Lederpedia.de besteht eine Zitierpflicht gemäß Lizenz CC Attribution-Share Alike 4.0 International. Informationen dazu finden Sie hier Zitierpflicht bei Verwendung von Inhalten aus Lederpedia.de. Für die kommerzielle Nutzung von Inhalten aus Lederpedia.de muss zuvor eine schriftliche Zustimmung (Anfrage via Kontaktformular) zwingend erfolgen.
www.Lederpedia.de - Lederpedia - Lederwiki - Lederlexikon
Eine freie Enzyklopädie und Informationsseite über Leder, Ledertechnik, Lederbegriffe, Lederpflege, Lederreinigung, Lederverarbeitung, Lederherstellung und Ledertechnologie